Histamine and Antihistamine ppt / Histamine and Antihistamine Classification / Histamine and Antihistamine Pharmacology / Histamine and Antihistamine Notes
HISTAMINE :
Histamine, meaning ‘tissue amine’ (histos—tissue) is almost ubiquitously present in animal
tissues and in certain plants, e.g. stinging nettle.
Its pharmacology was studied in detail by Dale
in the beginning of the 20th century when close
parallelism was noted between its actions and the
manifestations of certain allergic reactions. Histamine
was implicated as a mediator of hypersensitivity
phenomena and tissue injury reactions. It is now
known to play important physiological roles.
Histamine is present mostly within storage
granules of mast cells. Tissues rich in histamine
are skin, gastric and intestinal mucosa, lungs,
liver and placenta. Nonmast cell histamine
occurs in brain, epidermis, gastric mucosa
and growing regions. Turnover of mast cell
histamine is slow, while that of nonmast cell
histamine is fast. Histamine is also present
in blood, most body secretions, venoms and
pathological fluids.
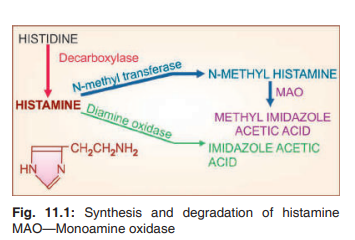
Synthesis, storage and destruction :
Histamine is β imidazolylethylamine. It is
synthesized locally from the amino acid histidine and degraded rapidly by oxidation and
methylation (Fig. 11.1). In mast cells, histamine
(positively charged) is held by an acidic protein
and heparin (negatively charged) within intracellular granules. When the granules are extruded
by exocytosis, Na+
ions in e.c.f. exchange with
histamine to release it free (Fig. 11.2). Increase
in intracellular cAMP (caused by β adrenergic
agonists and methylxanthines) inhibits histamine
release. Histamine is inactive orally because liver degrades all histamine that is absorbed
from the intestines.
Histamine receptors :
Four types of histaminergic receptors have now been clearly delineated
and cloned. Analogous to adrenergic α and β
receptors, histaminergic receptors were classified by Asch and Schild (1966) into H1
and
H2 :those blocked by then available antihistamines were labelled H1
. Sir James Black (1972)
produced the first H2
blocker burimamide and
confirmed this classification. Till now, only
these two receptors are clinically relevant. A
third H3
receptor, which serves primarily as
an autoreceptor controlling histamine release
from neurones in the brain was identified in
1983. Though some selective H3
agonists and
antagonists have been produced, none has found
any clinical application. Features of these 3
types of histaminergic receptors are compared
in Table 11.1.
Molecular cloning has revealed yet another (H4
) receptor
in 2001. It has considerable homology with H3
receptor
and binds many H3
ligands. 4-Methyl histamine, earlier In sensitized atopic individual, specific reaginic (IgE)
antibody is produced and gets bound to Fc epsilon receptor
I (FcεRI) on the surface of mast cells. On challenge, the
antigen bridges IgE molecules resulting in transmembrane
activation of a tyrosine-protein kinase (t-Pr-K) which phosphorylates and activates phospholipaseCγ. Phosphatidyl
inositol bisphosphate (PIP2
) is hydrolysed and inositol
trisphosphate (IP3
) is generated which triggers intracellular
release of Ca2+. The Ca2+ ions induce fusion of granule
membrane with plasma membrane of the mast cell resulting in exocytotic release of granule contents. In the granule,
positively charged histamine (Hist+
) is held complexed with
negatively charged protein (Prot–
) and heparin (Hep–
) molecules. Cationic exchange with extracellular Na+
(and Ca2+)
sets histamine free to act on the target cells.
PHARMACOLOGICAL ACTIONS :
1. Blood vessels :
Histamine causes marked
dilatation of smaller blood vessels, including arterioles, capillaries and venules. On s.c. injection flushing, especially in the blush area, heat,
increased heart rate and cardiac output, with
little or no fall in BP are produced. Rapid i.v.
injection causes fall in BP which has an early
short lasting H1
and a slow but more persistent
H2
component. With low doses only the H1
component is manifest since H1
receptors have
higher affinity. Fall in BP due to large doses
is completely blocked only by a combination
of H1
and H2
antagonists. Dilatation of cranial
vessels causes pulsatile headache.
Like many other autacoids and ACh, vasodilatation caused by histamine is partly (H1
component) indirect, mediated through ‘endothelium
dependent relaxing factor’ (EDRF), i.e. NO;
the receptor being located on the endothelial
cells. H2
receptors mediating vasodilatation are
located directly on the vascular smooth muscle.
Larger arteries and veins are constricted by
histamine. This is mediated by H1
receptor on
vascular smooth muscle. Histamine also causes
increased capillary permeability due to separation of endothelial cells resulting in exudation
of plasma. This is primarily a H1
response.
Injected intradermally, it elicits the triple
response consisting of:
Red spot: due to intense capillary dilatation.
Wheal: due to exudation of fluid from
capillaries and venules.
Flare: i.e. redness in the surrounding
area due to arteriolar dilatation
mediated by axon reflex.
2. Heart :
Direct effects of histamine on in situ
heart are not prominent, but the isolated heart,
especially of guinea pig, is stimulated—rate as
well as force of contraction is increased. These
are primarily H2
responses but a H1
mediated
negative dromotropic (slowing of A-V conduction) effect has also been demonstrated.
3. Visceral smooth muscle :
Histamine
causes bronchoconstriction; guinea pigs and
patients of asthma are highly sensitive. Large
doses cause abdominal cramps and colic by
increasing intestinal contractions. Guineapig
uterus is contracted while that of rat is relaxed;
human uterus is not much affected as are most
other visceral smooth muscles.
Smooth muscle contraction is a H1
response.
In few instances H2
mediated relaxation is also
seen, e.g. bronchial muscle of sheep, human
bronchi after H1
blockade when H2
response
is unmarked.
4. Glands :
Histamine causes marked increase
in gastric secretion—primarily of acid but also
of pepsin (see Ch. 47). This is a direct action
exerted on parietal cells through H2
receptors,
and is mediated by increased cAMP generation,
which in turn activates the membrane proton
pump (H+
K+
ATPase).
Histamine can increase other secretions also,
but the effect is hardly discernable.
5. Sensory nerve endings :
Itching occurs
when histamine is injected i.v. or intracutaneously. Higher concentrations injected more
deeply cause pain. These are reflections of the
capacity of histamine to stimulate nerve endings.
6. Autonomic ganglia and adrenal medulla :
These are stimulated and release of Adr
occurs, which can cause a secondary rise in BP.
7. CNS :
Histamine does not penetrate bloodbrain barrier—no central effects are produced on
i.v. injection. However, intracerebroventricular
administration produces rise in BP, cardiac
stimulation, behavioural arousal, hypothermia,
vomiting and ADH release. These effects are
mediated through both H1
and H2
postsynaptic
receptors.
PATHOPHYSIOLOGICAL ROLES :
1. Gastric secretion :
Histamine has dominant
physiological role in mediating secretion of
HCl in the stomach (see Fig. 47.1). Nonmast
cell histamine occurs in gastric mucosa, possibly in cells called ‘histaminocytes’ situated
close to the parietal cells. This histamine has
high turnover rate. It is released locally under
the influence of all stimuli that evoke gastric
secretion (feeding, vagal stimulation, cholinergic
drugs and gastrin) and activates the proton pump
(H+
K+
ATPase) through H2
receptors.
H2
blockers not only suppress acid secretion
induced by histamine but also markedly diminish that in response to ACh and gastrin. By a mutually synergistic interaction the three secretagogues
amplify responses to each other with histamine
playing the dominant role. As such, antimuscarinic
drugs dampen the response to histamine and
gastrin as well. All three secretagogues activate
the same proton pump (H+K+ATPase) in the
parietal cell membrane, but through their own
receptors.
2. Allergic phenomena :
Mediation of hypersensitivity reactions was the first role ascribed
to histamine. It is an important, but only one
of the mediators of such phenomena. Released
from mast cells following AG : AB reaction
on their surface (involving IgE type of reaginic
antibodies; Fig. 11.2) in immediate type of
hypersensitivity reactions, histamine is causative
in urticaria, angioedema, bronchoconstriction
and anaphylactic shock. The H1
antagonists are
effective in controlling these manifestations to
a considerable extent, except asthma and to a
lesser extent anaphylactic fall in BP in which
leukotrienes (especially LTD4
) and PAF appear
to be more important. Histamine is not involved
in delayed or retarded type of allergic reactions.
3. As transmitter :
Histamine is believed to be
the afferent transmitter which initiates the sensation of itch and pain at sensory nerve endings.
Nonmast cell histamine occurs in brain, especially hypothalamus and midbrain. It is involved
in maintaining wakefulness; H1
antihistaminics
owe their sedative action to blockade of this
function. In the brain H1
agonism suppresses
appetite. This may explain the appetite promoting
action of certain H1
antagonists. Histamine also
appears to participate as a transmitter regulating
body temperature, cardiovascular function, thirst,
and possibly other functions, mainly through
H2
(postsynaptic receptors) and H3
(presynaptic
autoreceptors and heteroreceptors).
4. Inflammation :
Histamine is a mediator
of vasodilatation and other changes that occur
during inflammation. It promotes adhesion of
leukocytes to vascular endothelium by expressing adhesion molecule P-selectin on endothelial
cell surface, sequestrating leukocytes at the inflammatory site. It may also regulate microcirculation according to local needs.
5. Tissue growth and repair :
Because growing and
regenerating tissues contain high concentrations of histamine, it has been suggested to play an essential role in
the process of growth and repair.
6. Headache :
Histamine has been implicated in certain
vascular headaches, but there is no conclusive evidence.
USES :
Histamine has no therapeutic use. In the past it has been
used to test acid secreting capacity of stomach, bronchial
hyperreactivity in asthmatics, and for diagnosis of pheochromocytoma, but these pharmacological tests are risky
and obsolete now.
Betahistine :
It is an orally active, somewhat
H1
selective histamine analogue, which is used to
control vertigo in patients of Meniéré’s disease.
It possibly acts by causing vasodilatation in the
internal ear. It is contraindicated in asthmatics
and ulcer patients.
HISTAMINE RELEASERS :
A variety of mechanical, chemical and immunological
stimuli are capable of releasing histamine from mast cells.
1. Tissue damage: trauma, stings and venoms, proteolytic
enzymes, phospholipase A.
2. Antigen: antibody reaction involving IgE antibodies.
3. Polymers like dextran, polyvinyl pyrrolidone (PVP).
4. Some basic drugs—tubocurarine, morphine, atropine,
pentamidine, polymyxin B, vancomycin and even some
antihistaminics release histamine by displacing it from
the binding site, and not by an immunological reaction.
This release is not exocytotic, and does not require Ca2+.
5. Surface acting agents like Tween 80, compound 48/80
etc. The primary action of these substances is release
of histamine from mast cells, therefore they are called
‘histamine liberators’. They produce an ‘anaphylactoid’
reaction—itching and burning sensation, flushing, urticaria,
fall in BP, tachycardia, headache, colic and asthma.
H1 ANTAGONISTS (Conventional antihistaminics) :
These drugs competitively antagonize actions of
histamine at the H1
receptors. Recent evidence
indicates that histamine H1
receptor exhibits some
degree of constitutive activity at certain sites, and
few H1
antagonists are also inverse agonists. The
first H1
antagonists were introduced in the late 1930s and have subsequently proliferated into
an unnecessary motley of drugs. Nevertheless,
they are frequently used for a variety of purposes. More commonly employed now are the
less sedating/nonsedating second generation H1
antihistamines added after 1980. Seemingly, H1
antihistaminics have diverse chemical structures,
but majority have a substituted ethylamine
side chain. They are classified and listed in
Table 11.2.
be continued ... next post
Histamine and Antihistamine ppt / Histamine and Antihistamine Classification / Histamine and Antihistamine Pharmacology / Histamine and Antihistamine Notes











gk ka notes laaoo
ReplyDelete